This article is an example of what I tutor in general chemistry. The size of an atom depends on the amount of electron subshells and how close the electrons are to the nucleus. The atomic radius increases going down the periods and decreases going from left to right.
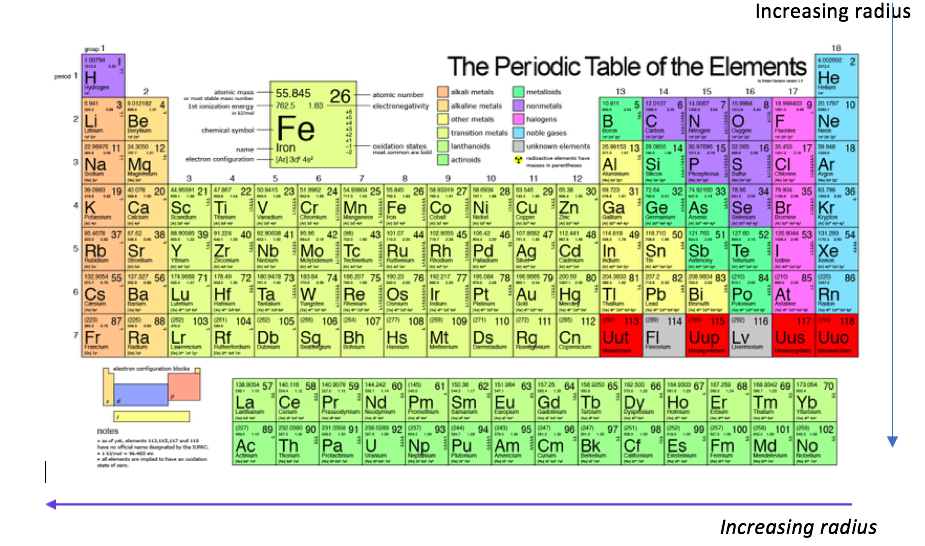
Nonbonding atomic radius – half of the shortest distance separating the two nuclei during gas collision.
Bonding atomic radius – half of the nucleus-to-nucleus distance in a molecular boding of two same atoms.
