This article is an example of what I tutor in general chemistry.
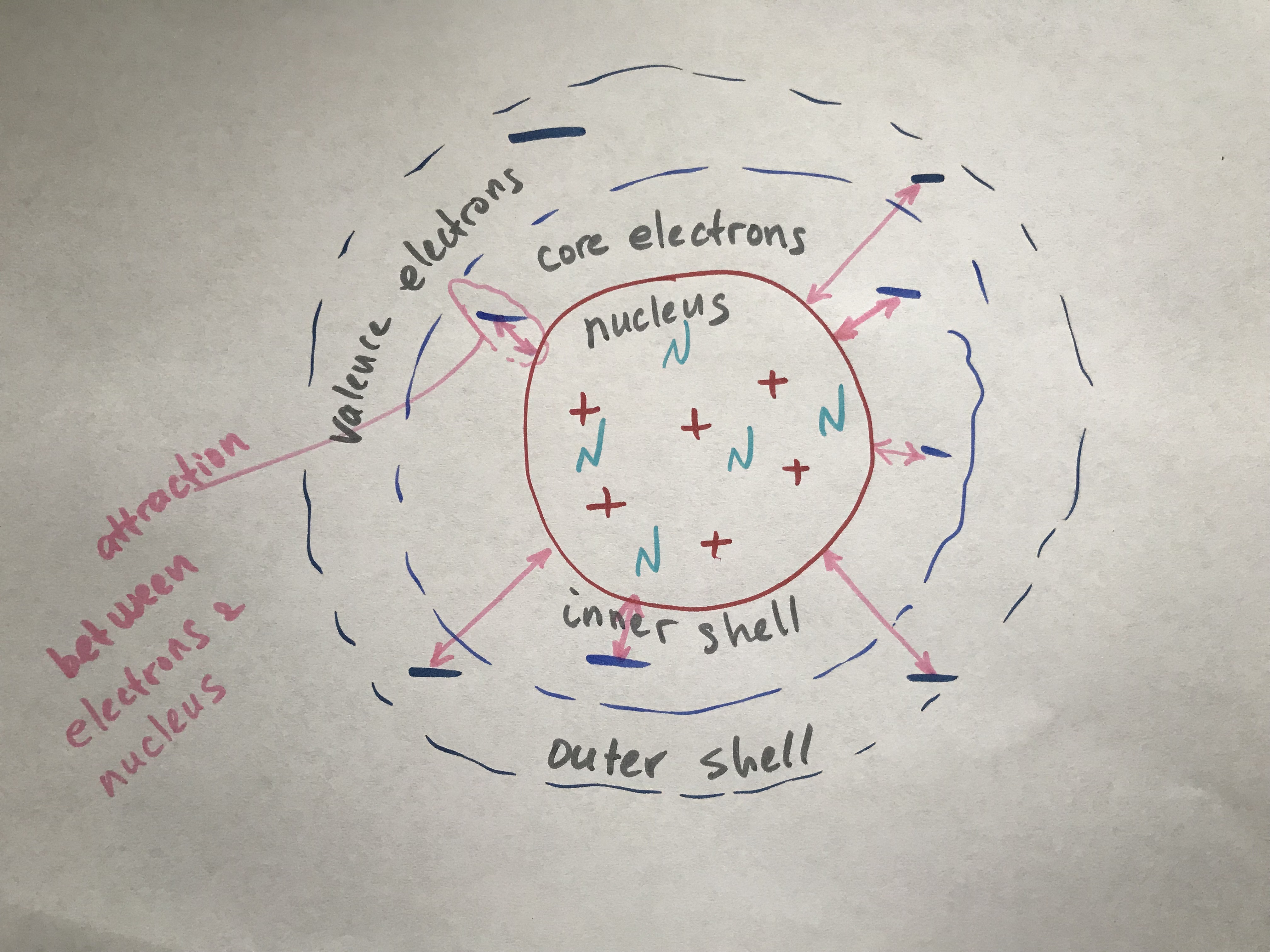
Nucleus of an atom is positively charged due to protons and neutrons composing its core. Protons are positive particles and neutrons are neutral particles. Hence, nucleus is positively charged due to protons’ positive charge. Electrons are negatively charged particles found on the outside of the nucleus. They are attracted to nucleus, since the opposite charges attract.
So the Effective Nuclear Charge (Zeff) is an attractive force between the electron and nucleus of an atom. It’s a positive charge that is acting on a single electron as a result of a positive charge located at the nucleus. It’s the force that is holding the electron in place next to the nucleus.
This attractive force between an electron and the nucleus depends on the magnitude of the nuclear charge and on the distance between the nucleus and the electron, per Coulomb’s Law. So, the further the electron away from the nucleus, the less attractive force is between it and the nucleus. This is why core electrons are harder to remove than valence electrons.
